AGC Chemicals Americas
-
 English
English
- Documentation
- Resources
- Blog
-
 Customer & product inquiries: 1-800-424-7833
Customer & product inquiries: 1-800-424-7833
AGC Chemicals Americas
 English
English
FORBLUE™ FLEMION™ is a fluorinated ion exchange membrane used to produce caustic soda/caustic potash in electrolysis plants. FLEMION membranes achieve substantial energy savings because they require less electrical current to decompose the purified brine. In addition to saving energy, FLEMION membranes minimize the influence of brine impurities and enable manufacturers to maintain high electrical current efficiency.
These membranes are used in the electrolyzers at electrolysis plants that decompose brine. They play a key part in manufacturing caustic soda (sodium hydroxide)/caustic potash (potassium hydroxide), chlorine, and hydrogen-basic chemical products. These chemicals are key ingredients of many products used in our daily lives.
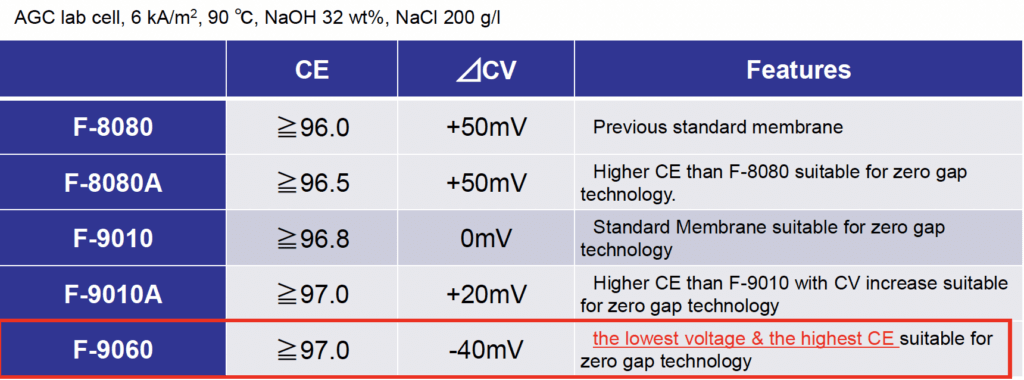
FLEMION F-9060 is the latest grade. It works at a much lower voltage than F-9010 (-40mV versus 0mV) and features a higher CE and other performance improvements making it the most suitable option for Zero-Gap Electrolyzer operations.
AGC has continued its efforts to reduce electric resistance of ion exchange membranes over the last 40 years by applying innovative designs. FLEMION is a fluorinated cation exchange membrane that offers extremely low resistance and is the preferred product by many customers.
Related Information